Wolfram Pönisch
How cell shapes change during cellular fate transitions
Keywords: theoretical biophysics, modelling, cell fate transitions, morphometrics, stochastic processes
The development of an organism relies on a series of cellular fate transitions where cells acquire increasingly specialized phenotypes. Cell fate changes have mostly been studied from the point of view of gene expression networks, with little attention paid to cell shape changes and the underlying mechanics that govern cell shapes. However, while there is evidence of feedbacks between cell shape and fate, shape changes during fate transitions have not been studied much, to a great extent due to a lack of tools to quantitively assess shape changes.
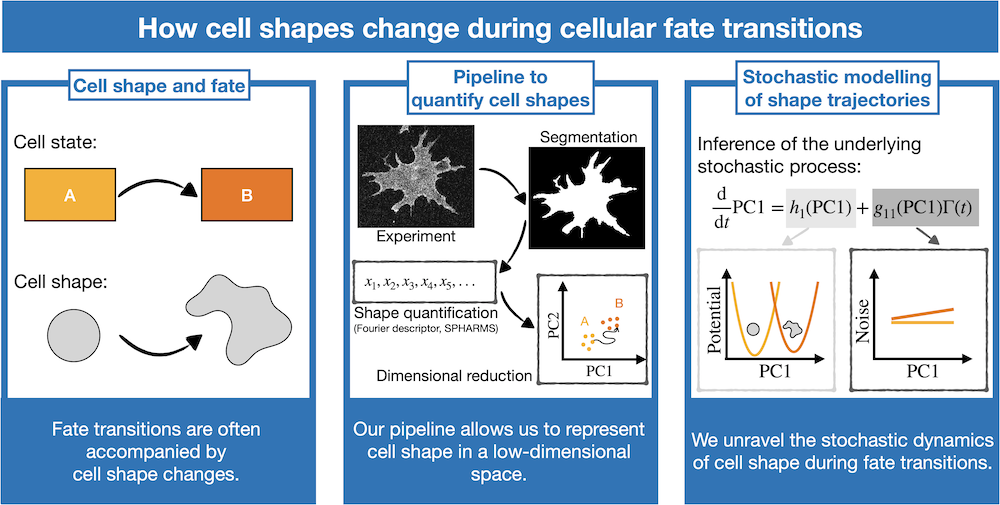
In my project, I study cellular shape changes and the coupling between cell shape and fate in various developmental contexts, as well as stem cells in cell culture where fate changes can be induced in a dish. I developed a pipeline for the quantification of cell shape during fate transitions. In close collaboration with experimentalists, the starting point of the
pipeline is the imaging of cells that undergo a fate transition and segmentation of cellular shapes with advanced machine learning algorithms. I then use mathematical shape descriptors (e.g. Fourier and spherical harmonic descriptors) to derive a collection of generic features that quantify cell shapes. This provides me with a high-dimensional data set that includes the complete shape information of a cell. To condense this extensive collection of data, in the final step of the pipeline I use dimensional reduction techniques that allow for the projection of the shape information into a low-dimensional space, called morphospace. Using this pipeline, I could characterise, in various biological contexts, the shape trajectories associated with fate transitions. For example, in close collaboration with the group of Dr. Elia Benito-Gutierrez from the Cambridge University Zoology department, we have investigated the cell shape changes underlying axis elongation during the development of amphioxus, an early chordate used as a model to gain insight into the evolution of vertebrates. We were not only able to identify the time-dependent shape trajectories of cells during embryo development but could also link cell shape to the location in the tissue and to overall tissue shape.
Next to unravelling which path cell shapes follow during fate transitions, my pipeline also enables me to study how robustly and reliably cells follow these developmental trajectories by quantifying the fluctuations of cell shapes. To this aim, I have been investigating the stochastic dynamics driving the cell shape trajectories during the “epithelial-to-mesenchymal transition”, a change in cell state underlying for instance cancer metastasis. I am collecting hundreds of shape trajectories for individual cells and infer the underlying stochastic process. This allows me to separate the potential landscape driving the shape transition and noise that emerges from shape and how both vary with time.
I believe that cell fate transitions cannot be understood without studying cell shape and mechanics. My work paves the way to the deciphering of the feedback between morphology and fate during development.
@WolframPonisch
Department of Physiology, Development and Neuroscience
Paluch Lab - @PaluchLab
Figure legend: Overview of the pipeline that allows me to investigate how cell shape changes during cell state transitions. This pipeline represents cell shape changes as trajectories in a low-dimensional representation. For the trajectories, I infer the underlying stochastic process to learn more about the physical processes driving cell state transitions.